Welcome to the SFABP Database!
This database provides unambiguous mass spectrometry evidence for the protein residue targets of SFABP, a sulfonyl fluoride activity-based probe designed to mimic the serine protease inhibitor AEBSF.
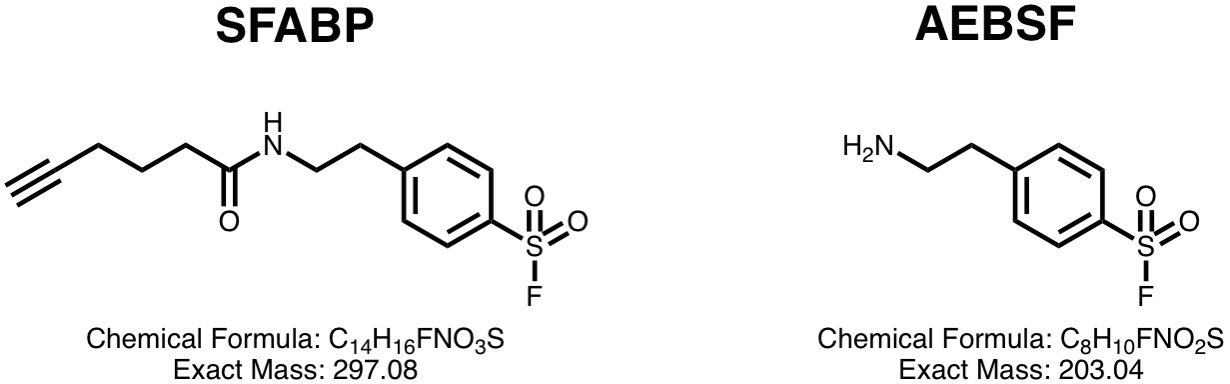
SUMMARY
SFABP was found to react with several lysine, tyrosine and serine residues on trypsin, chymotrypsin and GST. Unconstrained MS/MS sequencing identified that SFABP bound to the active site serine of trypsin (Ser200) is entirely displaced by hydrolysis, and heavy water labelling experiments elucidated two competing mechanisms.
REFERENCE
The entries of this database are directly hyperlinked from the following publication, where further details about the study can be found:
T.E.J. Chavas, M.J. Fuchter and P.A. DiMaggio Jr., "Unbiased Mass Spectrometry Elucidation of the Targets and Mechanisms of Activity-Based Probes: A Case Study involving Sulfonyl Fluorides", ACS Chemical Biology, 2018, doi: 10.1021/acschembio.8b00530